Sulphur Dioxide - Gas Profile




Sulphur Dioxide (SO2) is a heavy, colourless, poisonous gas that smells pungent - like a match when first lit and is deemed a toxic and corrosive chemical. Sulphur Dioxide is a combination of sulphur and oxygen created predominately through sulphuric acid manufacturing and combustion of sulphur fuels. It has a melting point of -75.5 Celcius and a boiling point of -10.
Applications such as coal and electric power plants, oil refineries and diesel automobile and machinery fumes contribute substantially to Sulphur Dioxide creation. Oil refinery start-ups, shutdowns and turnarounds have also been shown to increase levels. However, SO2 also exists naturally in volcanic gases, the water of warm springs in minimal levels of the atmosphere. Natural disasters such as forest fires have been proven to release significant levels of sulphur dioxide into the atmosphere. SO2, alongside Nitrogen dioxide and nitric oxide, is a major component of acid rain. SO2 can negatively affect humans through irritation to the eyes, nose and mouth, and symptoms can include coughing and choking.
Fun Fact – Sulphur Dioxide is used in agriculture to treat vegetables by destroying moulds and acting as a preservative

Applications used in
- Manufacturing industries – fertiliser, sulphuric acid to go into batteries and cleaners
- Food and Beveridge – food processing to dry food such as apricots and winemaking to protect the wine from spoiling – preservative and antioxidant
- Paper manufacturing – bleaching the paper.
- Agriculture - acts as a preservative and treatment
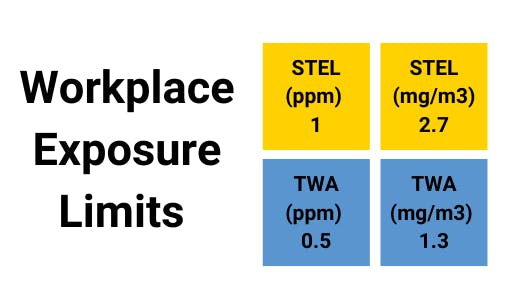
Assessment of the Dangerous Toxic Load (DTL) for Specified Level of Toxicity (SLOT) and Significant Likelihood of Death (SLOD)
- CAS number – 7446-09-5
- 'n' value - 2
- SLOT DTL (ppmn.min) – 4.655 x 10
- SLOD DTL (ppmn.min) – 7.448 x 10





