Hydrogen Fluoride - Gas Profile


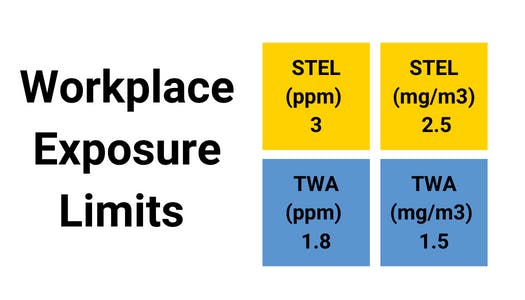


Hydrogen Fluoride (HF) is a fluorine-based chemical compound. It is a colourless gas, a fuming liquid, and a dissolvable solid in water. Even at low concentrations, hydrogen fluoride gas can irritate the eyes, nose, and lungs. Hydrogen fluoride can be fatal when inhaled at high concentrations or combined with skin contact, resulting in arrhythmia and pulmonary oedema.
Hydrogen Fluoride was discovered by the French chemist Edmond Frémy in the middle of the nineteenth century, while HF solutions had been known and used for at least a century before that. Nowadays, fluorite (calcium fluoride) is mined and processed to produce Hydrogen Fluoride gas using sulfuric acid. The mineral apatite, which includes fluoride ions and phosphate, is a significant source of HF produced as a byproduct of phosphate fertiliser production.
- Hydrofluoric acid is the name given to hydrogen fluoride when it is dissolved in water.
- Despite being the least dense of the hydrogen halides, it has the highest boiling point of the group.
- Refrigerants, herbicides, medicines, high-octane gasoline, aluminium, plastics, electrical components, and fluorescent light bulbs utilise hydrogen fluoride in their production.
- Sixty per cent of all industrial hydrogen fluoride consumption goes toward the production of refrigerants.
- Hydrogen Fluoride has a boiling point of 19.5 °C and a melting point of -83.37 °C
Fun Fact – Hydrogen Fluoride in its hydrofluoric acid form was used in the TV series Breaking Bad.

Applications used in
- Refrigerant Production
- Petrochemical
- Metal and Plastic Works
- Pharmaceuticals
- Electrical Component Production